Ibyingenzi bidasanzwe byubutaka: Ni ubuhe buryo bukoreshwa nifu ya yttrium oxyde?
Isi idasanzwe ni umutungo wingenzi cyane, kandi ifite uruhare rudasubirwaho mubikorwa byinganda.Ibirahuri byimodoka, magnetiki resonance resonance, fibre optique, kwerekana amazi ya kirisiti yerekana, nibindi ntibishobora gutandukana no kongeramo isi idasanzwe.Muri byo, yttrium (Y) ni kimwe mu bintu bidasanzwe byubutaka bwisi kandi ni ubwoko bwicyuma.Nyamara, kubera ibiyirimo byinshi mubutaka bwisi, igiciro kirahendutse kandi kirakoreshwa cyane.Mu musaruro rusange urimo, ukoreshwa cyane cyane muri leta ya yttrium alloy na yttrium oxyde.
Yttrium Metal
Muri byo, okiside yttrium (Y2O3) ningirakamaro cyane yttrium.Ntishobora gushonga mumazi na alkali, gushonga muri acide, kandi ifite isura yifu ya kirisiti yera (imiterere ya kirisiti ni sisitemu ya cubic).Ifite imiti ihamye cyane kandi iri mu cyuho.Ihindagurika rito, irwanya ubushyuhe bwinshi, irwanya ruswa, dielectric nyinshi, gukorera mu mucyo (infrared) nibindi byiza, bityo yagiye ikoreshwa mubice byinshi.Nibihe byihariye? Reka turebe.
Imiterere ya kristu ya yttrium oxyde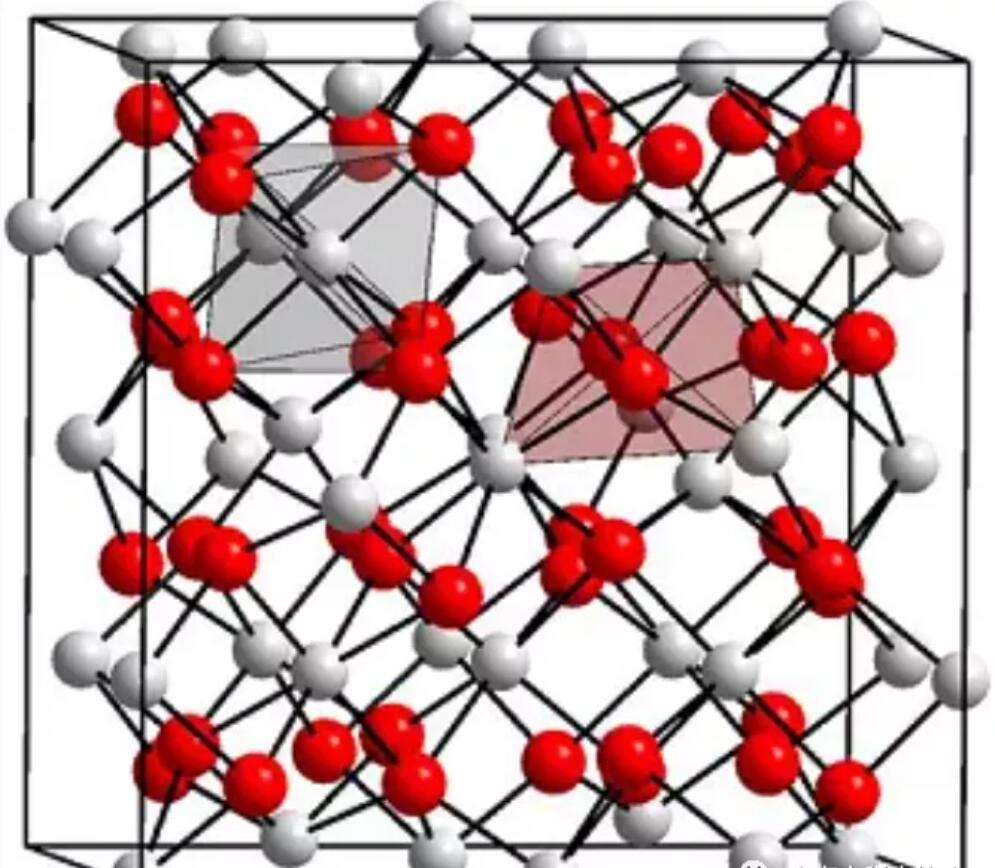
01 Synthesis ya yttrium ituje ifu ya zirconia.Icyiciro gikurikira kizahinduka mugihe cyo gukonjesha kwa ZrO2 kuva ubushyuhe bwo hejuru kugeza ubushyuhe bwicyumba: icyiciro cya cubic (c) phase tetragonal phase (t) phase icyiciro cya monoclinike (m), aho t izabera kuri 1150 ° C → m ihinduka ryicyiciro, biherekejwe no kwagura amajwi agera kuri 5%.Ariko, niba icyiciro cya t → m cyerekezo cya ZrO2 gihindagurika kubushyuhe bwicyumba, inzibacyuho ya t → m iterwa no guhangayika mugihe cyo gupakira. Bitewe ningaruka zijwi ryatewe nimpinduka zicyiciro, imbaraga nyinshi zavunitse zirakirwa , kugirango ibikoresho bigaragaze imbaraga zidasanzwe zidasanzwe, kuburyo ibikoresho byerekana ubukana buvunitse budasanzwe, bikaviramo gukomera kwicyiciro, no gukomera kwinshi no kwihanganira kwambara cyane.igitsina.
Kugirango ugere ku cyiciro cyo guhindura ubukana bwa ceramika ya zirconi, hagomba kongerwaho stabilisateur kandi mugihe runaka cyo kurasa, ubushyuhe bwo hejuru butajegajega-tetragonal meta-stabilisation yubushyuhe bwicyumba, bukabona icyiciro cya tetragonal gishobora guhinduka mugice cyubushyuhe bwicyumba .Ningaruka zifatika za stabilisateur kuri zirconiya.Y. yakwegereye abantu benshi.02 Imfashanyigisho zo gucumura Gucumura ibintu byinshi byihariye byubutaka bisaba uruhare rwibikoresho byo gucumura.Uruhare rwibikoresho byo gucumura rushobora kugabanywamo ibice bikurikira: gukora igisubizo gihamye hamwe nicyaha; Irinde guhinduka kwa kristu;kubuza imikurire ya kristu;kubyara icyiciro cyamazi.Kurugero, mugucumura alumina, magnesium oxyde MgO ikunze kongerwaho nka microstructure stabilisateur mugihe cyo gucumura.Irashobora gutunganya ibinyampeke, kugabanya cyane itandukaniro ryingufu zimbibi zimbibi, kugabanya anisotropy yo gukura kwingano, kandi bikabuza gukura kwimbuto.Kubera ko MgO ihindagurika cyane ku bushyuhe bwo hejuru, kugirango tugere ku bisubizo byiza, okiside Yttrium ikunze kuvangwa na MgO.Y2O3 irashobora gutunganya ibinyampeke bya kirisiti kandi igateza imbere gucumura.03YAG ifu ya syntetique yttrium aluminium garnet (Y3Al5O12) nikintu cyakozwe numuntu, nta minerval naturel, ibara ritagira ibara, ubukana bwa Mohs bushobora kugera kuri 8.5, gushonga 1950 ℃, kudashonga muri acide sulfurique, aside hydrochloric, aside nitric, aside hydrofluoric, nibindi. ubushyuhe bwo hejuru icyiciro gikomeye nuburyo bwa gakondo bwo gutegura ifu ya YAG. Ukurikije igipimo cyabonetse mubishushanyo mbonera cya binary igishushanyo cya yttrium oxyde na oxyde ya aluminium, ifu zombi zivanze kandi zirasa ku bushyuhe bwinshi, kandi ifu ya YAG ikorwa binyuze mu bikomeye -icyiciro cya reaction hagati ya oxyde.Mugihe cy'ubushyuhe bwo hejuru, mubisubizo bya alumina na yttrium oxyde, mesofase YAM na YAP bizabanza gukorwa, hanyuma amaherezo YAG.
Ubushyuhe bwo hejuru-uburyo bukomeye bwo gutegura ifu ya YAG ifite porogaramu nyinshi.Kurugero, ingano ya Al-O ingano ni nto kandi ingufu zububiko ni nyinshi.Ingaruka za electron, imikorere ya optique igumaho itajegajega, kandi kwinjiza ibintu bidasanzwe byubutaka birashobora kuzamura cyane imikorere ya luminescence ya fosifore.Kandi YAG irashobora guhinduka fosifore mugukoporora hamwe na ion zidasanzwe zidasanzwe nka Ce3 + na Eu3 +.Mubyongeyeho, YAG kristal ifite umucyo mwiza, imiterere ihamye yumubiri na chimique, imbaraga za mashini nyinshi, hamwe no guhangana nubushyuhe bwiza.Nibikoresho bya laser kristu hamwe nurwego runini rwa porogaramu nibikorwa byiza.
YAG kristu 04 ibonerana ceramic yttrium oxyde yamye nubushakashatsi bwibanze mubijyanye nubutaka buboneye.Nibintu bya cubic sisitemu kandi ifite isotropique optique ya buri murongo.Ugereranije na anisotropy ya alumina ibonerana, ishusho ntigoretse, kuburyo buhoro buhoro, yagiye ihabwa agaciro kandi itezwa imbere na lens zohejuru cyangwa idirishya rya gisirikari.Ibintu nyamukuru biranga imiterere yumubiri nubumashini ni: pointIcyerekezo cyo hejuru cyo gushonga, Imiterere ya chimique na fotokome ni byiza, kandi optique ya optique iragutse (0.23 ~ 8.0μm);TKuri 1050nm, igipimo cyayo cyo kwanga kiri hejuru ya 1.89, bigatuma igira ihererekanyabubasha rirenga 80%;③Y2O3 ifite ibihagije kugirango ihuze byinshi Umuyoboro utandukanya umurongo munini uva kumurongo munini ugera kumurongo wa valence urwego rwohereza imyuka yubutaka butatu bwisi bushobora guhuzwa neza na doping ya ion zidasanzwe zisi.None rero kugirango tumenye imikorere myinshi ikoreshwa. ;EnergyIngufu za fonon ziri hasi, kandi numubare munini wa fonon ucamo ni 550cm-1.Ingufu nke za fonon zirashobora guhagarika amahirwe yo guhinduka kutari imirasire, kongera amahirwe yo guhinduranya imirasire, no kunoza kwantumasi ya luminescence;⑤Ubushyuhe bukabije bwumuriro, hafi 13.6W / (m · K), ubushyuhe bwinshi bwumuriro burakabije
ingenzi kuri yo nkibikoresho bikomeye bya laser.
Yttrium oxide ceramics ibonerana yatunganijwe nu Buyapani Kamishima Chemical Company
Ingingo yo gushonga ya Y2O3 ni 2690 ℃, naho ubushyuhe bwo gucengera mubushyuhe bwicyumba ni 1700 ~ 1800 ℃.Gukora ububumbyi bwohereza urumuri, nibyiza gukoresha imashini zishyushye no gucumura.Bitewe nubwiza buhebuje bwumubiri nubumashini, Y2O3 yubukorikori bubonerana burakoreshwa cyane kandi bushobora gutezwa imbere, harimo: amadirishya ya misile infrarafarike na domes, lens zigaragara kandi zitagira infrarafuriya, amatara yohereza gaze yumuvuduko mwinshi, ceramic scintillator, laseri ceramic nizindi mirima
Igihe cyo kohereza: Ugushyingo-25-2021





