Shin kun sani? Tsarin mutane na ganowayttriumya cika da swists da kalubale. A cikin 1787, Axel Axel Arrenius ya gano wani yanki mai yawa da kuma mai suna kusa da ƙauyen daga garinsu, kuma sunanta shi ". Bayan haka, masana kimiyya da yawa ciki har da Johan Gaddolin, Anders Gustav Ekberg, Friedrich Wöhler da wasu sun yi bincike mai zurfi a kan wannan titin.
A cikin 1794, Chemist na Chemist na Chemen Chemist Johan Gadolin ya yi nasarar samun sabon okide daga yetsgium ore da kuma sanya shi shi yttrium. Wannan shi ne karo na farko da mutane suka gano a fili cewa mutane a fili ya gano wani abu mai wuya duniya. Koyaya, wannan gano bai jawo hankalin da yadu da kai tsaye ba.
A tsawon lokaci, masana kimiyya sun gano wasu abubuwan duniya da yawa. A cikin 1803, KLAproth da Swedes Hitzinger da Berzelius sun gano Cerium. A cikin 1839, Swede Mosander ya ganoLanthanum. A cikin 1843, ya gano erbium daterbium. Wadannan binciken sun samar da ingantaccen tushe don binciken kimiyya na gaba.
Ba har zuwa ƙarshen karni na 19 ba cewa masana kimiyya sunyi nasarar raba kashi "Yttrium" daga YTTRIUM. A cikin 1885, Austrian Wilsbach ya gano Neodlium da Pratsardmium. A cikin 1886, Bois-Baudran ya ganodyspsprosum. Wadannan binciken ya kara wadatar da babban gidan duniya abubuwan duniya.
Fiye da karni bayan ganowa na YTTRrium, saboda iyakokin yanayin fasaha, masana kimiya sun kasa yanke hukunci da wannan siginan, wanda ya haifar da wasu rigakafin ilimi da kurakurai. Koyaya, wannan bai daina masana kimiyya daga kwazonsu don nazarin yttrium.
A farkon karni na 20, tare da ci gaba da cigaban cigaban kimiyya da fasaha, a ƙarshe masana kimiyya suka fara iya tsarkake abubuwan duniya. A cikin 1901, Faransa Eugene de Marseille ya ganokuɗin kuɗin shiga. A cikin 1907-1908, Austrian Wilsbach da Faransa urbain da kansa aka gano Lutetium. Wadannan binciken sun samar da ingantaccen tushe don binciken kimiyya na gaba.
A cikin kimiyya da fasaha na zamani, aikace-aikacen YTTTrium ya zama mafi yawa da yawa. Tare da cigaban cigaban kimiyya da fasaha, fahimtarmu da aikace-aikacen YTTrium zai zama mafi zurfin zurfin ciki.
Filayen aikace-aikace na kayan yamma
1.Gilashin Optical da Bratinics:Ana amfani da Yttrium sosai a cikin keɓaɓɓen gilashin gani da kuma bernication, galibi a cikin samarwa na Brarrics da Gilashin Optical. Hukumar sa suna da kyawawan kaddarorin pictical kuma ana iya amfani dasu don ƙirƙirar kayan haɗin laser, fiber-optic sadarwa da sauran kayan aiki.
2. Proosphors:Abubuwan da ke cikin YTTTrium suna taka muhimmiyar rawa a cikin phospfors kuma zasu iya fitar da haske mai haske mai haske, saboda haka ana amfani dasu don samarwa TV na TV, masu saka idanu da kayan aiki.Yttrium oxideKuma ana amfani da sauran mahadi azaman kayan lumingen don haɓaka haske da haske mai haske.
3. Awoy ƙari: A cikin samar da filayen ƙarfe, ana amfani da yttrium a matsayin ƙari don inganta kaddarorin injin da juriya na morrous.Yattrium Aloyana amfani da shi sau da yawa don yin ƙarfi-ƙarfiAlumuran aluminium, sa su more zafi-resistant da lalata juriya.
4. Catalysts: Hyprium mahadi yana taka muhimmiyar rawa a wasu masu kara kuzari kuma suna iya hanzarta yawan halayen sunadar sunadarai. Ana amfani da su don haɓaka na'urorin tsarkakewa na motoci da masu biyan kuɗi a cikin matakan samarwa na masana'antu, suna taimakawa rage faɗuwar abubuwa masu cutarwa.
5. Fasaha na Maryuwa: Ana amfani da istrium a fasahar yin amfani da likita don shirya kayan motsa jiki, kamar don shimfida radiopharmicals da bincike na likita.
6. Fasaha Laser:Laser na YTTTRUIIS IONS ne na kowa mai ƙarfi-jihar Laser da aka yi amfani da shi a cikin binciken kimiyya, likitocin laser da aikace-aikacen masana'antu. Da samarwa daga cikin waɗannan lasers na buƙatar amfani da wasu mahadi na ƙwayoyin Yttrium a matsayin masu fafutuka.Yttrium abubuwaKuma mahaɗan su taka muhimmiyar rawa a kimiyyar zamani da fasaha da masana'antu, sun ƙunshi filaye da dama kamar su ci gaba da haɓaka jama'ar mutane.
Kayan jiki na Yttrium
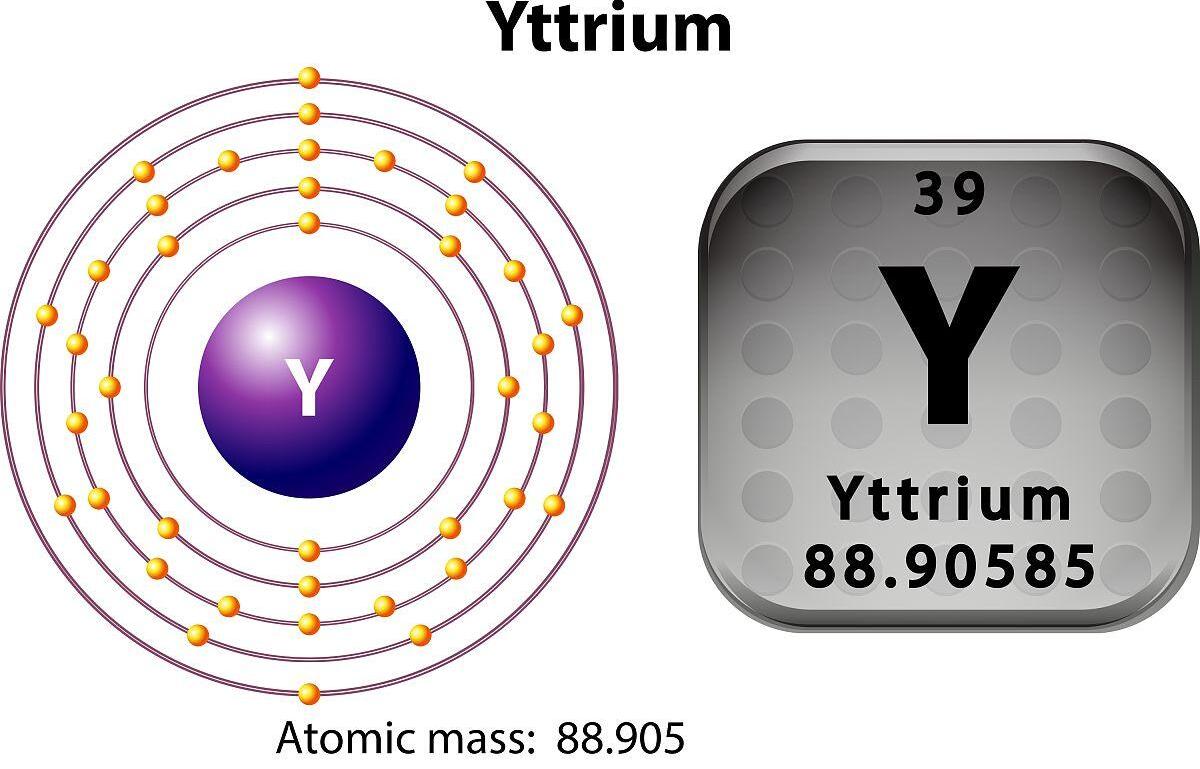
A atomic Yawanyttriumshine 39 da alamar sunadarai shine y.
1. Bayyanar:Yttrium wani farin ƙarfe ne-fari.
2. Yawan:Yawan Yttrium shine 4.47 g / cm3, wanda ya sa ya zama mai nauyi a cikin ɓawon burodi na duniya.
3. Meming Point:Matsayin narkewa na YTTTrium shine 15 Digiri Celsius (2782 digiri Fahrenshet), wanda ke nufin zazzabi wanda ya canza zuwa wani ruwa na yttrium daga wani ruwa a ƙarƙashin yanayin zafi.
4. Burin tafasa:Matsayin tafasasshen yttrium shine 3336 digiri Celsius (6037 digiri Fahrenshet), wanda ke nufin yawan zafin jiki wanda ya canza zuwa ruwa zuwa gas a ƙarƙashin yanayin zafi.
5. Lokaci:A zazzabi a dakin, yttrium yana cikin m jihar.
6. Yin magana:Yttrium mai jagoranci ne na wutar lantarki tare da babban aiki, saboda haka yana da wasu aikace-aikace a cikin masana'antar masana'antun lantarki da fasahar da'ira.
7. Magnetism:Yttrium kayan abu ne na paramagnetic a zazzabi a daki, wanda ke nufin cewa ba shi da bayyananniyar magana game da magnetic filayen magnetic.
8. Tsarin Crystal: Yttrium ya wanzu a cikin hexagonal tsarin crystal.
9. Yawan Atomic:A attom girma na YTTRIUM 19.8 Cubic santimita a kowace gular, wanda ke nufin ƙara da tawaya yttrium atoms.
Yttrium adadi mai ƙarfe ne tare da babban yawa da narkewarsa, kuma yana da kyawawan aikace-aikace na mahimmanci a cikin lantarki, Kayan Aiki da sauran filayen. A lokaci guda, YTTRIUS ita ce wani abu mai wuya ne na yau da kullun, wanda ke taka muhimmiyar rawa a wasu manyan fasahohin haɓaka da aikace-aikace masana'antu.
Kayan sunadarai na yttrium
1. Alamar kemikal da rukunin sunadarai: Alamar sunadarai na Yttrium shine Y, kuma tana cikin tebur na lokaci-lokaci, rukuni na uku, wanda ya yi kama da abubuwan Lanthanii.
2. Electronic structure: The electronic structure of yttrium is 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d¹⁰ 4f¹⁴ 5s². A cikin Layer Layer, Yttrium yana da wayoyin lantarki biyu.
3. Jihar Varece: YTTRIIIL yawanci yana nuna yanayin Varence na +3, wacce ita ce babbar jihar Valence, amma kuma tana iya nuna mahimman jihohin +2 da +1.
4. Haɗuwa: Yttrium shine tsayayyen ƙarfe, amma zai sannu a hankali ya fallasa iska, samar da shimfidar wuri a farfajiya. Wannan yana haifar da yttrium don rasa luster. Don kare Yttrium, yawanci ana adana shi ne a cikin yanayin bushewa.
5. Dauki tare da okides: Yttrium sake da okes don samar da mahadi daban-daban, ciki har dayttrium oxide(Y2o3). Sau da yawa ana amfani da oxide oxide don yin phospors da yerammens.
6. ** dauki da acid **: Yttrium na iya amsawa da karfi acid don samar da salts masu dacewa, kamarYttrium chloride (YCL3) koyttrium sulfate (Y2 (so4) 3).
7. Dauki tare da ruwa: Yttrium bai amsa kai tsaye da ruwa na al'ada ba, zai iya amsawa da tururi na ruwa da yttrium oxide.
8. Dauki tare da sulfies da carbies: yttrium na iya amsawa da sulfes da carbriumes mai dacewa (yt) da kuma yttrium carbide (yc2). 9. Isotopes: Yettrium yana da isotopes da yawa, mafi kyawun abin da yake shine yttrium-89 (^ 89y), wanda ke da dogon rayuwa a cikin maganin nukiliya da ake amfani da shi.
Yttrium wani abu ne mai tsayayye mai tsayayye tare da kasashe masu yawa da yawa da kuma ikon yin amsawa tare da wasu abubuwan don samar da mahadi. Tana da kewayon aikace-aikace da yawa a cikin ɗabi'a, magani, da masana'antu, musamman ma a cikin furofesoshin, yumbu masana'antu.
Abubuwan ilimin halittu na ilimin halitta na yttrium
Abubuwan halittu nayttriumA cikin halittu masu rai suna da iyaka.
1. Kasance da ingession: Kodayake yttrium ba mahimmanci bane ga rayuwa, gano adadin YTTTrium cikin yanayi, gami da ƙasa, duwatsu, da ruwa. Kwayoyiniyoyi na iya shiga cikin alamar yttrium ta hanyar sarkar abinci, yawanci daga ƙasa da tsire-tsire.
2. Idiavailability: The rioavailability na YTTrium ba low, wanda ke nufin cewa kwayoyin gaba ɗaya suna da wahalar shayar da amfani da yttrium yadda ya kamata. Yawancin mahadi na yttrium ba a sauƙin tunawa a cikin kwayoyin, don haka sai suka watsar da su.
3. Rarraba a cikin kwayoyin: sau ɗaya a cikin kwayoyin, an rarraba Yttrium a cikin kyallen takarda kamar hanta, koda, daji, huhu, da kasusuwa. Musamman, kasusuwa suna dauke da manyan taro na yttrium.
4. Metabolism da excrovation: metabolism na yttrium a cikin jikin mutum yana da iyaka saboda yawanci yana barin kwayoyin da extretion. Yawancin shi ana rufe su ta hanyar fitsari, kuma ana iya cire shi ta hanyar gamawa.
5. Kayayyaki: Saboda ƙarancin ƙarfinsa, Yttrium baya tara matakan cutarwa a cikin kwayoyin. Koyaya, bayyanar girman-kashi na yiwuwar yiwuwar cutarwa akan kwayoyin halitta, yana haifar da tasirin guba. Wannan yanayin yawanci yakan faru da wuya saboda taro na ytttrium a cikin yanayin yawanci ba shi da yawa kuma ba a yi amfani da shi ba. Dukda cewa bashi da mummunan sakamako masu guba akan kwayoyin halittu a ƙarƙashin yanayi na yau da kullun, babban-kashi ana iya haifar da haɗarin lafiya. Saboda haka, binciken kimiyya da sa ido har yanzu suna da mahimmanci ga aminci da tasirin ilimin halittu na YTTRIUM.
Rarraba YTTTRIUM a cikin yanayi
Yttrium wani abu ne mai wuya duniya wanda ya kasance in mun gwada da yadu sosai a cikin yanayi, ko da yake ba ya wanzu a cikin kyakkyawan tsari.
1. Aukuwa a cikin ɓawon burodi na duniya: Yawan yttrium a cikin ɓawon burodi na ƙasa ya kasance low, tare da maida hankali da kimanin 33 mg / kg. Wannan ya sa yttrium daya daga cikin abubuwan da ke da wuya.
Yttrium yafi ya wanzu ne ta hanyar ma'adanai, yawanci tare da sauran abubuwan duniya da yawa. Wasu manyan ma'adinai sun hada da yttrium Ironar Garnet (YAG) da yttrium okalate (y2 (c2o4) 3).
2. Rarraba Gerographical: ana rarraba adibas a duk faɗin duniya, amma wasu yankuna na iya zama mai arziki a cikin yttrium. Ana iya samun wasu manyan ajiya na ajiya a yankuna masu zuwa: Ostiraliya, China, Kanada, India, Scandinavia, da zarar ana buƙatar Kanada, India, Scandinavia, yawanci ana buƙatar Kanada, da zarar ana buƙatar Kanada, da zarar ana buƙatar sarrafa yttrium don cirewa kuma suna raba yttrium. Wannan yawanci ya shafi leaching da acid leaching da rabuwa na sinadarai don samun tsarkakakken tsarkin yttrium.
Yana da mahimmanci a lura cewa abubuwa masu karancin ƙasa kamar su yttrium ba sau da yawa suna wanzu a cikin sifofin tsarkakewa, amma an gauraye da sauran karnukan duniya. Saboda haka, hakar mafi girma yttrium na buƙatar hadaddun ke da sinadarai da tafiyar matakai. Bugu da kari, wadatarAbubuwa na Duniyayana da iyaka, saboda la'akari da aikinsu na kayan aikin su da dorewa mai dorewa shine kuma mahimmanci.
Ma'adinai, hakar da smtlting na kashi yttrium
Yttrium wani abu ne mai wuya a duniya wanda yawanci ba ya wanzu a cikin hanyar tsarkakakken yttrium, amma a cikin hanyar yttrium ore. Mai zuwa cikakken bayani ne ga ma'adinan mintuna da kuma gyara tsarin YTTTRIUM.
1. Mining na YTTRIUM ORT:
Binciken: na farko, na ilimin halittu da injiniyoyin ma'adinai suna gudanar da aikin bincike don nemo adiban da ke dauke da yttrium. Wannan yawanci ya ƙunshi karatun halittu, binciken ra'ayi, da bincike na samfurin. A haƙa: da zarar an sami ajiya wanda ya samo yttrium, ore mined. Wadannan adibas suna yawanci hada da ores kamar kaztrium iron garnet (Yig) ko yttrium (y2 (c2o4) 3). Ore murkushe: Bayan hadi, da ore yakan buƙatar karye shi cikin ƙananan guda don aiki mai zuwa.
2. Cire yttrium:Leach Leaching: yawanci ana tura shi zuwa smelter, inda aka fitar da yttrium ta hanyar leaching leaching. Wannan tsari yawanci yana amfani da maganin leachic na acidic, kamar sulfuric acid, don narke yttrium daga nttrium daga ore. Rarraba: Da zarar an narkar da Yttrium, galibi ana hade shi da sauran karancin duniya da impurities. Don cire yttrium na mafi girma tsarkakakku, ana buƙatar tsari rabuwa, yawanci amfani da hakar mai yawa, ion musayar ko wasu hanyoyin sunadarai. Yanayi: Yettrium ya rabu da sauran karancin duniya Abubuwa ta hanyar halayen sinadarai masu dacewa don samar da tsarkakakken mahadi da yttrium. Bushewa da lissafi: 'Yan mahaifa da aka samu yawanci suna buƙatar bushewa da callsed don cire duk wani danshi na danshi da a ƙarshe samun ƙaho na ƙarfe ko ƙwayoyin cuta.
Gano hanyoyin YTTTRIUM
Hanyoyin ganowa gama gari ne na YTTRIIISHE sun hada da wasan kwaikwayo na atomroscy (AAS), wanda aka haɗa shi da hannu, X-Ms), x-ray cryorescence Spectroscopy (X-Ray), da dai sauransu.
1Aas ne da aka saba amfani da tsarin bincike da yawa da aka saba amfani da shi don tantance abun cikin YTTRIUS a cikin bayani. Wannan hanyar ta dogara ne akan tunanin lokacin sha yayin da manufa ta kasance a cikin samfurin yana shan hasken takamaiman igiyar ruwa. Da farko, an canza samfurin zuwa wani abu mai kyau ta hanyar presreatment matakai kamar bushewa da bushe-zafi bushe. Bayan haka, Haske wanda ya dace da raƙuman zartarwar da aka yi amfani da shi a cikin samfurin, hasken wutar lantarki ana lissafta shi tare da daidaitaccen samfurin yttrium na sananniyar taro.
2.ICP-MS shine babban dabarar bincike mai mahimmanci don tantance abun cikin YTTRIUL a cikin ruwa da kuma samfurori mai ƙarfi. Wannan hanyar tana sauya samfurin a cikin barbashi mai caji sannan kuma yayi amfani da taro na mai bincike ga masu bincike. ICP-MS yana da kewayon kewayon ganowa da babban ƙuduri, kuma na iya tantance abubuwan da yawa na abubuwa da yawa a lokaci guda. Don gano YTTRIUM, ICP-MS na iya samar da iyakance da ƙananan daidaito da babban daidaito.
3. X-ray Cryorescence Spectometry (XRF):Xrf hanya ce mai lalacewa wacce ta dace ta dace da tabbacin abun ciki na Yttrium a cikin samfuran ruwa mai ƙarfi da ruwa. Wannan hanyar tana tantance asalin abun ciki ta hanyar irradiating saman samfurin tare da X-haskoki da kuma auna halayen kamun ƙwararraki a cikin samfurin. XRF yana da fa'idodi na saurin sauri, aiki mai sauƙi, da kuma ikon sanin abubuwa da yawa a lokaci guda. Koyaya, ana iya yin amfani da XRF tare da bincike na ƙananan abun ciki yttrium, wanda ya haifar da manyan kurakurai.
4.Yana kula da playma playma opiction spectrometery shine mai matukar hankali da hankali da kuma zaban hanyar bincike sosai a cikin binciken da yawa. Yana atomizes samfurin da samar da plasma don auna takamaiman yanayin da kuma tsanani of yttriumwatsi a cikin specrometer. Baya ga hanyoyin da ke sama, akwai wasu hanyoyin da aka saba amfani da su don ganowa na YTTT, da sauransu kayan ganima, da kuma ƙa'idodin ganowa da kuma daidaitattun hanyoyin da ake buƙata don tabbatar da daidaito da amincin sakamako.
Takamaiman aikace-aikace na hanyar tsayayyar hanyar amfani
A cikin kashi biyu na kashi, a hankali hade plasma taro m taro specrometry (ICP-MS) Ma'anar bincike mai mahimmanci da yawa, wanda ake yawan amfani da shi don tantance maida hankali, ciki har da yttrium. Mai zuwa cikakken tsari ne don gwada yttrium a ICP-MS:
1. Shiri Samfura:
Samfuran yawanci yana buƙatar narkar da ko watsa shi cikin tsarin ruwa don bincike na ICP-MS. Za'a iya yin wannan ta hanyar rushewar sunadarai, yana narkar da narkewa ko sauran hanyoyin shirye-shiryen da suka dace.
Shirya samfurin yana buƙatar yanayi mai tsabta don hana gurbatawa ta kowane abu na waje. Dole ne dakin binciken ya dauki matakan da suka wajaba don gujewa gurbata samar da samfurin.
2. IcP tsara:
Ana samar da ICP ta hanyar gabatar da Argon ko Argon-Oxygen Has a cikin rufaffiyar ma'adini Plasma Torch. High-Bi-da yawa hada kai yana samar da harshen wuta mai zurfi, wanda shine farkon bincike.
A zazzabi na plasma kusan digiri 800 zuwa 10000 zuwa digiri na 10000, wanda ya isa ya canza abubuwan a cikin samfurin zuwa jihar ionic.
3. Ionizzation da rabuwa:Da zarar samfurin ya shiga plasma, abubuwan a ciki sun i ionized. Wannan yana nuna cewa kwayoyin zarra sun yi asara ɗaya ko fiye da wutan lantarki, suna haifar da cajin ions. ICP-MS yayi amfani da taro mai fa'ida don ware ions na abubuwa daban-daban, yawanci ta hanyar caja rabo (m / z). Wannan yana ba da damar ous na abubuwa daban-daban da za a rabu kuma daga baya aka bincika.
4. Taro na farko:Ya rabu ions shigar da taro Spectrometer, yawanci wani zuadrupole taro spectrometer ko kuma subning taro spectrometer. A cikin taro spectrometer, da aka ware wasu abubuwa daban-daban kuma an gano su gwargwadon cajin su. Wannan yana ba da damar kasance tare da maida hankali ga kowane abu. Daya daga cikin fa'idodin da aka hada shi da hannu plactrometry na farko shine babban ƙuduri, wanda ke ba da damar gano abubuwa da yawa lokaci guda.
5. Gudanar da bayanai:Data da aka kirkira ta ICP-MS yawanci ana buƙatar aiwatar da shi kuma ana bincika shi don sanin maida hankali a cikin samfurin. Wannan ya hada da kwatanta siginar ganowa ga ka'idojin sanannun taro, da kuma yin kalaman daidaitawa da gyara.
6. Sakamakon Rahoton:Sakamakon ƙarshe an gabatar dashi azaman taro ko kashi ɗaya daga cikin kashi. Ana iya amfani da waɗannan sakamakon a aikace-aikace iri iri, gami da kimiyyar duniya, gwajin na abinci, bincike na abinci, da sauransu.
ICP-MS ingantacciya ce mai mahimmanci kuma mai hankali ya dace da bincike mai yawa, ciki har da yttrium. Koyaya, yana buƙatar takaddara mai rikitarwa da ƙwarewa, don haka ana yin shi a cikin dakin gwaje-gwaje ko cibiyar bincike ta kwararru. A cikin ainihin aiki, ya zama dole a zaɓi hanyar ma'aunin da ta dace gwargwadon lokacin da shafin yanar gizon. Waɗannan hanyoyin ana amfani da su sosai a cikin bincike da kuma gano yadda ya faɗi a cikin dakunan gwaje-gwaje da masana'antu.
Bayan taƙaita abubuwan da ke sama, zamu iya yanke hukuncin cewa Yttrium mai ban sha'awa ne mai ban sha'awa tare da keɓaɓɓun kaddarorin kayan aiki, wanda yake da muhimmanci sosai a cikin binciken kimiyya da ayyukan aikace-aikace. Kodayake mun sami ci gaba a cikin fahimtarmu, har yanzu akwai tambayoyi da yawa da suke buƙatar ƙarin bincike da bincike. Ina fatan gabatarwarmu na iya taimaka wa masu karatu su fahimci wannan kashi mai ban sha'awa da kuma wahayi soyayya ga kimiyya da sha'awar yin bincike.
Don ƙarin bayani plsTuntube muA ƙasa:
Tel & menene: 008613524231522
Email:Sales@shxlchem.com
Lokacin Post: Nuwamba-28-2024