Ifu ya Cobalt Oxide Co3O4

Ibisobanuro
1.Izina: nanoCobalt OxideIfu ya Co3O4
2.Ubuziranenge: 99.9% min
3.Appearacne: ifu yumukara wumukara
4.Ubunini bw'igice: 50nm
5.SSA: 30-80 m2 / g
Ibyiza:
Guhura n'umwuka, byoroshye gukuramo ubuhehere, ariko ntibibyara amazi.Irashobora gushonga muri acide ya nitric.Iyo ishyutswe hejuru ya 1200 oC, oxyde ya nano-cobalt izacika mo oxyde-cobalt.Mu kirimi cya hydrogène, oxyde ya nano-cobalt ishyutswe kugeza kuri 900 oC, izahinduka cobalt yicyuma.Cobalt (II, III) oxyde ni imiti ivanze na formula Co3O4.Numukara ukomeye, hamwe nuruvange rwa valence ivanze, irimo Co (II) na Co (III) okiside yerekana.Irashobora gutegurwa nka CoIICoIII2O4 cyangwa CoO.Co2O3.Okiside ya Cobalt (II), CoO, ihinduka kuri Co3O4 iyo ishyushye kugeza kuri 600-700 ° C mu kirere.Hejuru ya 900 ° C, CoO irahagaze.
Gusaba:
Catalizike, superconductor, ceramics nizindi nzego nkibikoresho byingenzi bidasanzwe;Nka catalizator na catalizike itwara hamwe na electrode ikora;Kubirahuri, amabara ya farashi na pigment;Inganda zikora imiti oxyde na catalizike ya synthesis;Amadarubindi akomeye nibindi bikoresho byo kuyungurura;Carbide;Ubushyuhe na gaze;Inganda ziciriritse, ubukorikori bwa elegitoronike, ibikoresho bya electrode ya lithium ion, ibikoresho bya magneti;Ibikoresho by'amashanyarazi;Enamels;Gusya;Heterogeneous catalizator;Imirasire y'izuba ....
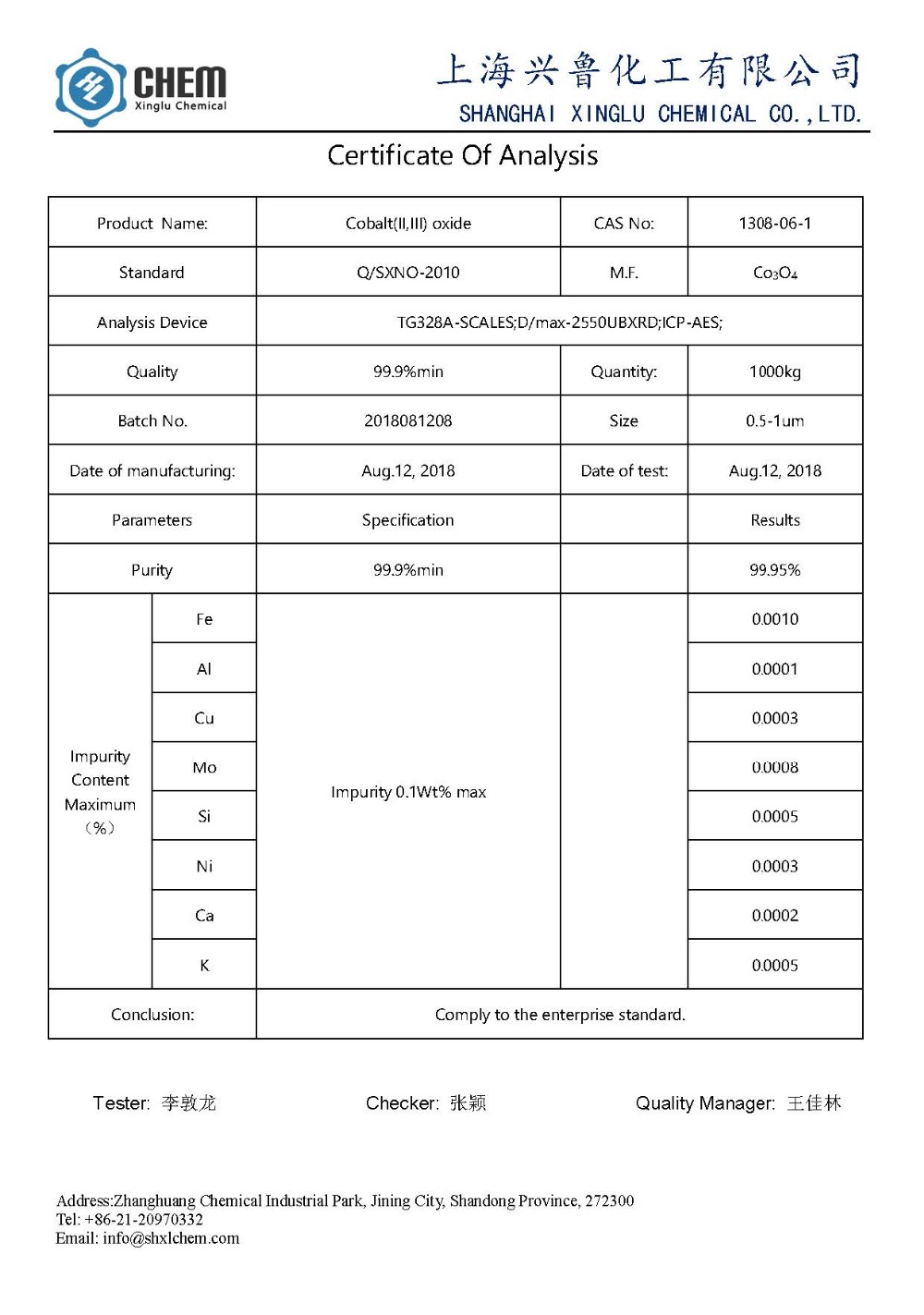
Icyemezo:

Icyo dushobora gutanga: