Cobalt oxide Co3O4 phofo

Tlhaloso
1.Lebitso: nanoCobalt oxideCo3O4 phofo
2.Bohloeki: 99.9% min
3.Appearacne: phofo e bohlooho e ntšo
4.Particle boholo: 50nm
5.SSA: 30-80 m2/g
Thepa:
Ho pepesehela moea, ho bonolo ho monya mongobo, empa ha e hlahise metsoako ea metsi.E qhibiliha ka asiti ea nitric.Ha mocheso o ka holimo ho 1200 oC, nano-cobalt oxide e tla robeha ho sub-cobalt oxide.Ka lelakabe la hydrogen, nano-cobalt oxide e futhumala ho 900 oC, e tla fetoloa hore e be cobalt ea tšepe.Cobalt(II,III) oxide ke motsoako oa lik'hemik'hale o nang le foromo ea Co3O4.Ke motsoako o motšo, le motsoako oa valence o tsoakiloeng, o nang le linaha tse peli tsa Co (II) le Co (III).E ka etsoa e le CoIICoIII2O4 kapa CoO.Co2O3.Cobalt(II) oxide, CoO, e fetohela ho Co3O4 haeba e futhumatsoa ho isa ho 600-700 °C moeeng.Ka holimo ho 900 °C, CoO e tsitsitse.
Kopo:
Catalysis, superconductors, ceramics le masimo a mang e le thepa ea bohlokoa ea inorganic;E le catalyst le catalyst carrier le eleketrode e sebetsang lintho tse bonahalang;Bakeng sa khalase, mebala ea porcelain le li-pigments;Oxidant ea indasteri ea lik'hemik'hale le mohloli oa motsoako oa manyolo;Li-goggles tse kholo le lisebelisoa tse ling tsa filthara;Carbide;Lisebelisoa tsa mocheso le khase;Bakeng sa indasteri ea semiconductor, ceramic ceramics, lithium ion battery electrode materials, magnetic materials;Lisebelisoa tsa electrochromic;Li-enamel;Mabili a sila;Heterogeneous catalysts;Lisebelisoa tsa matla a letsatsi ....
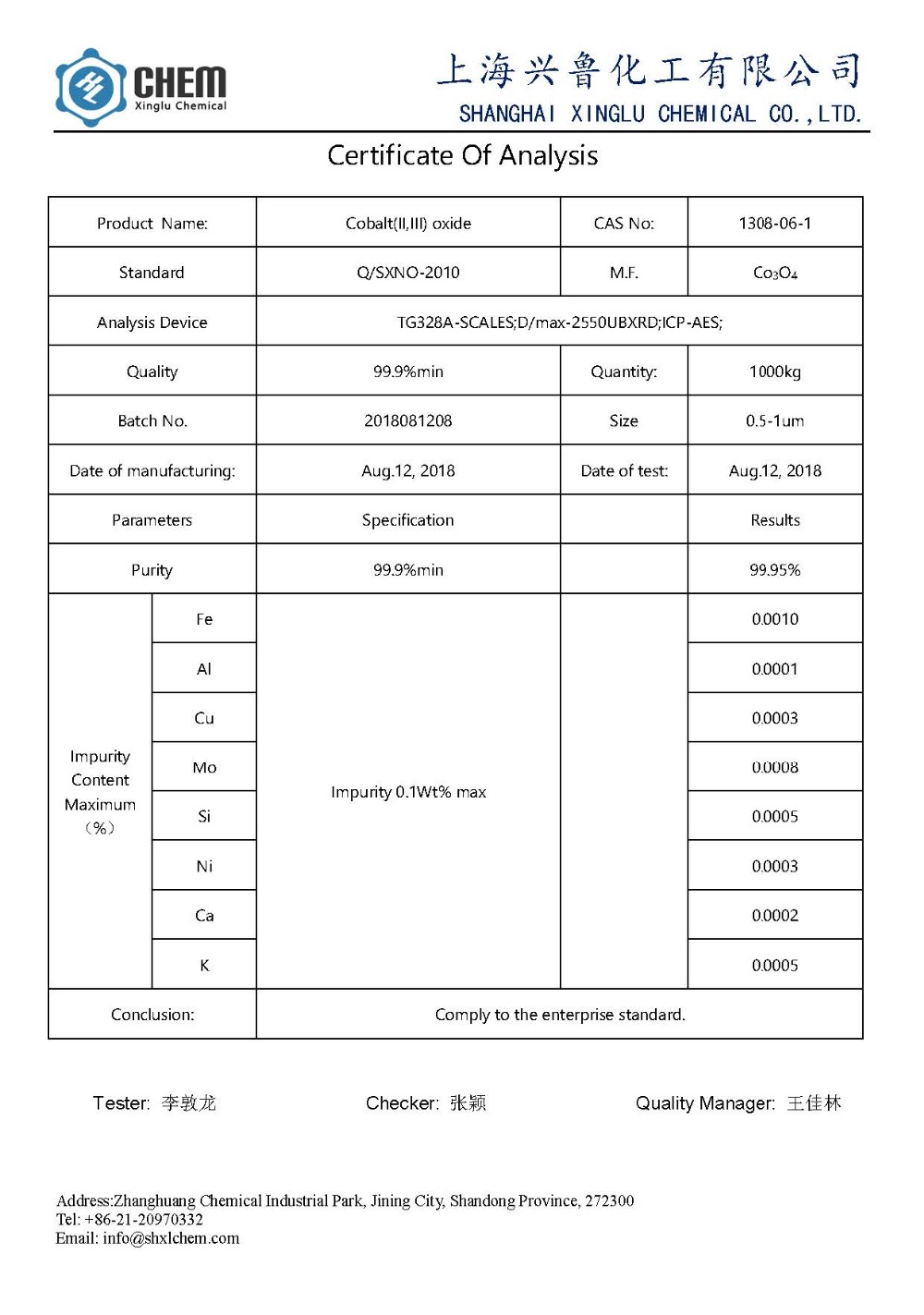
Setifikeiti:

Seo re ka fanang ka sona: