Cobalt Oxide Co3O4 pauka

Hōʻike
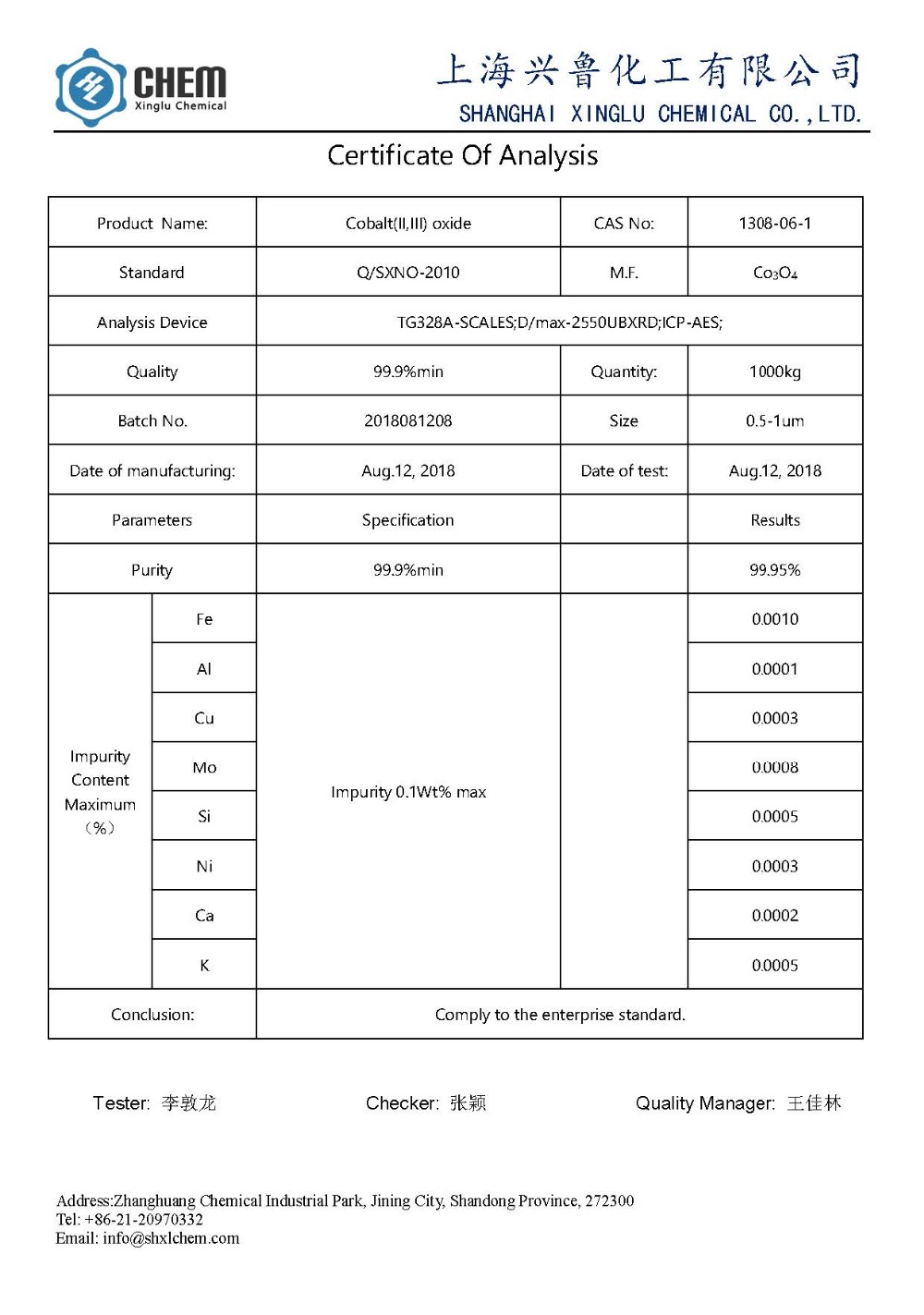
1.Inoa: nanoʻO ka ʻOxide CobaltCo3O4 pauda
2. Maʻemaʻe: 99.9% min
3.Appearacne: hina ʻeleʻele pauka
4.Particle nui: 50nm
5.SSA: 30-80 m2 / g
Waiwai:
ʻO ka ʻike i ka ea, maʻalahi ke komo i ka makū, akā ʻaʻole e hoʻohua i nā pūhui wai.Hiki ke hoʻoheheʻe ʻia i ka waikawa nitric.Ke hoʻomehana ʻia ma luna o 1200 oC, e wāwahi ʻia ka nano-cobalt oxide i loko o ka sub-cobalt oxide.I loko o ka lapalapa hydrogen, hoʻomehana ʻia ka nano-cobalt oxide i 900 oC, e hoʻololi ʻia ia i ka cobalt metala.ʻO ka Cobalt(II,III) oxide ka hui kemika me ke ʻano Co3O4.He paʻa ʻeleʻele ia, a he hui valence hui pū ʻia, loaʻa nā mokuʻāina ʻokiʻoki ʻelua Co(II) a me Co(III).Hiki ke hana ʻia e like me CoIICoIII2O4 a i ʻole CoO.Co2O3.Hoʻololi ʻo Cobalt(II) oxide, CoO, i Co3O4 inā wela a ma kahi o 600-700 °C i ka lewa.Ma luna o 900 °C, paʻa ka CoO.
Noi:
Catalysis, superconductors, ceramics a me nā māla ʻē aʻe ma ke ʻano he mea koʻikoʻi inorganic;E like me ka catalyst a me ka mea lawe catalyst a me ka mea hana electrode;No ke aniani, nā kala porcelain a me nā puaʻa;ʻO ka ʻoihana kemika a me ka mea hoʻoheheʻe no ka synthesis organik;ʻO nā maka aniani kiʻekiʻe a me nā mea kānana ʻē aʻe;Carbide;Nā meaʻike wela a me ke kinoea;No ka ʻoihana semiconductor, nā mea uila uila, lithium ion battery electrode material, magnetic material;Nā mea uila electrochromic;Nā enamel;wili huila;Nā mea hoʻoheheʻe heterogeneous;Nā mea hoʻopau ikehu lā....
Palapala:

ʻO ka mea hiki iā mākou ke hāʻawi: