CeO2 is an important component of rare earth materials. The rare earth element cerium has a unique outer electronic structure - 4f15d16s2. Its special 4f layer can effectively store and release electrons, making cerium ions behave in the+3 valence state and+4 valence state. Therefore, CeO2 materials have more oxygen holes, and have excellent ability to store and release oxygen. The mutual conversion of Ce (III) and Ce (IV) also endows CeO2 materials with unique oxidation-reduction catalytic capabilities. Compared to bulk materials, nano CeO2, as a new type of inorganic material, has received widespread attention due to its high specific surface area, excellent oxygen storage and release ability, oxygen ion conductivity, redox performance, and high-temperature rapid oxygen vacancy diffusion ability. There are currently a large number of research reports and related applications using nano CeO2 as catalysts, catalyst carriers or additives, active components, and adsorbents.
1. Preparation method of nanometer cerium oxide
At present, the common preparation methods for nano ceria mainly include chemical method and physical method. According to different chemical methods, chemical methods can be divided into precipitation method, hydrothermal method, solvothermal method, sol gel method, microemulsion method and electrodeposition method; The physical method is mainly the grinding method.
1.1 Grinding method
The grinding method for preparing nano ceria generally uses sand grinding, which has the advantages of low cost, environmental friendliness, fast processing speed, and strong processing ability. It is currently the most important processing method in the nano ceria industry. For example, the preparation of nano cerium oxide polishing powder generally adopts a combination of calcination and sand grinding, and the raw materials of cerium based denitration catalysts are also mixed for pre-treatment or treated after calcination using sand grinding. By using different particle size sand grinding bead ratios, nano ceria with D50 ranging from tens to hundreds of nanometers can be obtained through adjustment.
1.2 Precipitation method
The precipitation method refers to the method of preparing solid powder by precipitation, separation, washing, drying, and calcination of raw materials dissolved in appropriate solvents. The precipitation method is widely used in the preparation of rare earth and doped nanomaterials, with advantages such as simple preparation process, high efficiency, and low cost. It is a commonly used method for preparing nano ceria and its composite materials in industry. This method can prepare nano ceria with different morphology and particle size by changing the precipitation temperature, material concentration, pH value, precipitation speed, stirring speed, template, etc. Common methods rely on the precipitation of cerium ions from ammonia generated by urea decomposition, and the preparation of nano ceria microspheres is controlled by citrate ions. Alternatively, cerium ions can be precipitated by OH - generated from the hydrolysis of sodium citrate, and then incubated and calcined to prepare flake like nano ceria microspheres.
1.3 Hydrothermal and solvothermal methods
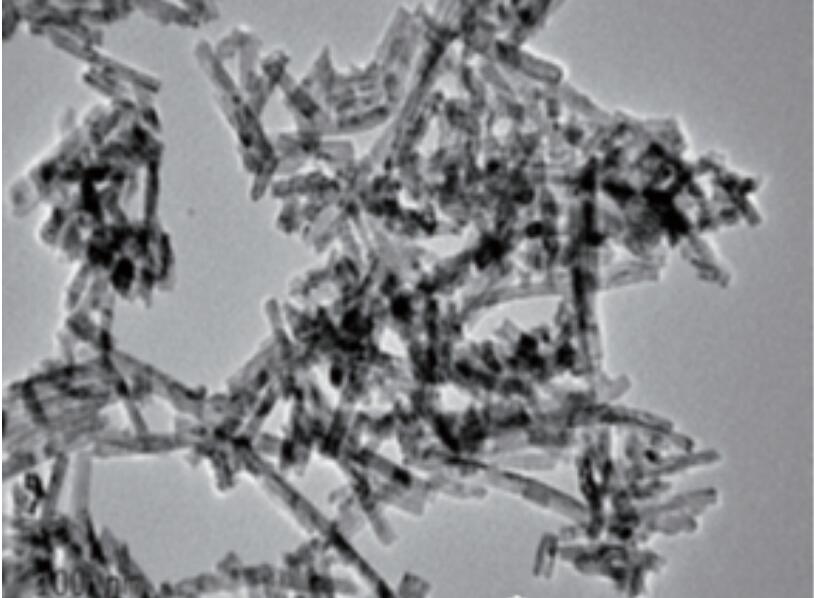
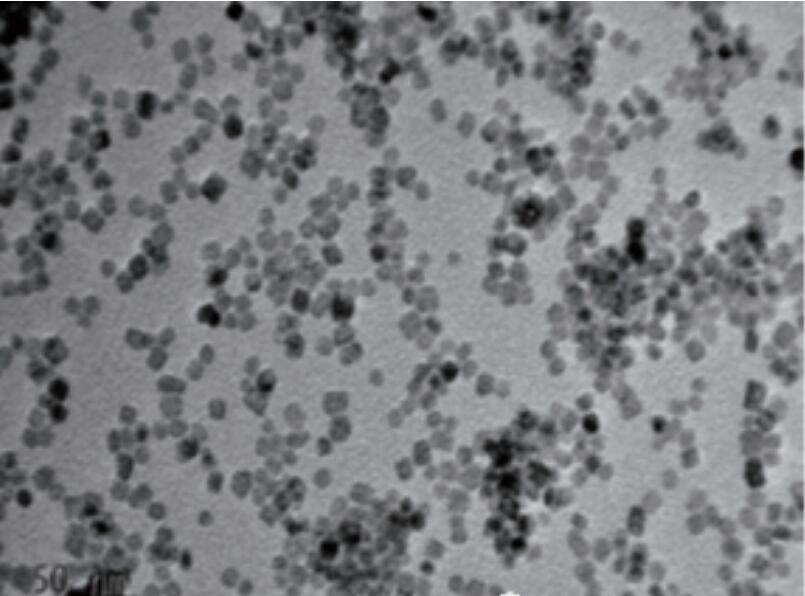
These two methods refer to the method of preparing products by high-temperature and high-pressure reaction at critical temperature in a closed system. When the reaction solvent is water, it is called hydrothermal method. Correspondingly, when the reaction solvent is an organic solvent, it is called solvothermal method. The synthesized nano particles have high purity, good dispersion and uniform particles, especially the nano powders with different morphologies or exposed special crystal faces. Dissolve cerium chloride in distilled water, stir and add sodium hydroxide solution. React hydrothermal at 170 ℃ for 12 hours to prepare cerium oxide nanorods with exposed (111) and (110) crystal planes. By adjusting the reaction conditions, the proportion of (110) crystal planes in the exposed crystal planes can be increased, further enhancing their catalytic activity. Adjusting the reaction solvent and surface ligands can also produce nano ceria particles with special hydrophilicity or lipophilicity. For example, adding acetate ions to the aqueous phase can prepare monodisperse hydrophilic cerium oxide nanoparticles in water. By selecting a non-polar solvent and introducing oleic acid as a ligand during the reaction, monodisperse lipophilic ceria nanoparticles can be prepared in non-polar organic solvents. (See Figure 1)
Figure 1 Monodisperse spherical nano ceria and rod-shaped nano ceria
1.4 Sol gel method
The sol gel method is a method that uses some or several compounds as precursors, conducts chemical reactions such as hydrolysis in the liquid phase to form sol, and then forms gel after aging, and finally drys and calcines to prepare ultrafine powders. This method is particularly suitable for preparing highly dispersed multi-component nano ceria composite nanomaterials, such as cerium iron, cerium titanium, cerium zirconium and other composite nano oxides, which have been reported in many reports.
1.5 Other methods
In addition to the above methods, there are also micro lotion method, microwave synthesis method, electrodeposition method, plasma flame combustion method, ion-exchange membrane electrolysis method and many other methods. These methods have great significance for the research and application of nano ceria.
Application of 2-nanometer cerium oxide in water treatment
Cerium is the most abundant element among rare earth elements, with low prices and wide applications. Nanometer ceria and its composites have attracted much attention in the field of water treatment due to their high specific surface area, high catalytic activity and excellent structural stability.
2.1 Application of Nano Cerium Oxide in Water Treatment by Adsorption Method
In recent years, with the development of industries such as the electronics industry, a large amount of wastewater containing pollutants such as heavy metal ions and fluorine ions has been discharged. Even at trace concentrations, it can cause significant harm to aquatic organisms and the human living environment. Commonly used methods include oxidation, flotation, reverse osmosis, adsorption, nanofiltration, biosorption, etc. Among them, adsorption technology is often adopted due to its simple operation, low cost, and high treatment efficiency. Nano CeO2 materials have high specific surface area and high surface activity as adsorbents, and there have been many reports on the synthesis of porous nano CeO2 and its composite materials with different morphologies to adsorb and remove harmful ions from water.
Research has shown that nano ceria has strong adsorption capacity for F - in water under weak acidic conditions. In a solution with an initial concentration of F - of 100mg/L and pH=5-6, the adsorption capacity for F - is 23mg/g, and the removal rate of F - is 85.6%. After loading it onto a polyacrylic acid resin ball (loading amount: 0.25g/g), the removal ability of F - can reach over 99% when treating an equal volume of 100mg/L of F - aqueous solution; When processing 120 times the volume, more than 90% of F - can be removed. When used to adsorb phosphate and iodate, the adsorption capacity can reach over 100mg/g under the corresponding optimal adsorption state. The used material can be reused after simple desorption and neutralization treatment, which has high economic benefits.
There are many studies on the adsorption and treatment of toxic heavy metals such as arsenic, chromium, cadmium, and lead using nano ceria and its composite materials. The optimal adsorption pH varies for heavy metal ions with different valence states. For example, the weak alkaline condition with neutral bias has the best adsorption state for As (III), while the optimal adsorption state for As (V) is achieved under weak acidic conditions, where the adsorption capacity can reach over 110mg/g under both conditions. Overall, the optimized synthesis of nano ceria and its composite materials can achieve high adsorption and removal rates for various heavy metal ions over a wide pH range.
On the other hand, cerium oxide based nanomaterials also have outstanding performance in adsorbing organics in wastewater, such as acid orange, rhodamine B, Congo red, etc. For example, in existing reported cases, nano ceria porous spheres prepared by electrochemical methods have high adsorption capacity in the removal of organic dyes, especially in the removal of Congo red, with an adsorption capacity of 942.7mg/g in 60 minutes.
2.2 Application of nano ceria in Advanced oxidation process
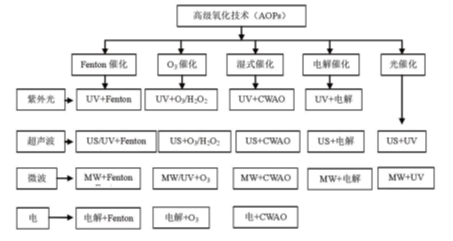
Advanced oxidation process (AOPs for short) is proposed to improve the existing anhydrous treatment system. Advanced oxidation process, also known as deep oxidation technology, is characterized by the production of hydroxyl radical (· OH), superoxide radical (· O2 -), singlet oxygen, etc. with strong oxidation ability. Under the reaction conditions of high temperature and pressure, electricity, sound, light irradiation, catalyst, etc. According to the different ways of generating free radicals and reaction conditions, they can be divided into photochemical oxidation, catalytic wet oxidation, sonochemistry oxidation, ozone oxidation, electrochemical oxidation, Fenton oxidation, etc. (see Figure 2).
Figure 2 Classification and Technology Combination of Advanced oxidation process
Nano ceria is a heterogeneous catalyst commonly used in Advanced oxidation process. Due to the rapid conversion between Ce3+and Ce4+and the rapid oxidation-reduction effect brought about by oxygen absorption and release, nano ceria has good catalytic ability. When used as a catalyst promoter, it can also effectively improve catalytic ability and stability. When nano ceria and its composite materials are used as catalysts, the catalytic properties vary greatly with the morphology, particle size, and exposed crystal planes, which are key factors affecting their performance and application. It is generally believed that the smaller the particles and the larger the specific surface area, the more corresponding active site, and the stronger the catalytic ability. The catalytic ability of the exposed crystal surface, from strong to weak, is in the order of (100) crystal surface>(110) crystal surface>(111) crystal surface, and the corresponding stability is opposite.
Cerium oxide is a semiconductor material. When nanometer cerium oxide is irradiated by photons with energy higher than the band gap, the valence band electrons are excited, and the transition recombination behavior occurs. This behavior will promote the conversion rate of Ce3+and Ce4+, resulting in strong photocatalytic activity of nano ceria. Photocatalysis can achieve direct degradation of organic matter without secondary pollution, so its application is the most studied technology in the field of nano ceria in AOPs. At present, the main focus is on the catalytic degradation treatment of azo dyes, phenol, chlorobenzene, and pharmaceutical wastewater using catalysts with different morphologies and composite compositions. According to the report, under the optimized catalyst synthesis method and catalytic model conditions, the degradation capacity of these substances can generally reach more than 80%, and the removal capacity of Total organic carbon (TOC) can reach more than 40%.
Nano cerium oxide catalysis for the degradation of organic pollutants such as ozone and hydrogen peroxide is another widely studied technology. Similar to photocatalysis, it also focuses on the ability of nano ceria with different morphologies or crystal planes and different cerium based composite catalytic oxidants to oxidize and degrade organic pollutants. In such reactions, catalysts can catalyze the generation of a large number of active radicals from ozone or hydrogen peroxide, which attack organic pollutants and achieve more efficient oxidative degradation capabilities. Due to the introduction of oxidants in the reaction, the ability to remove organic compounds is greatly enhanced. In most reactions, the final removal rate of the target substance can reach or approach 100%, and the TOC removal rate is also higher.
In the electrocatalytic advanced oxidation method, the properties of the anode material with high oxygen evolution overpotential determine the selectivity of the electrocatalytic advanced oxidation method for treating organic pollutants. The cathode material is an important factor determining the production of H2O2, and the production of H2O2 determines the efficiency of the electrocatalytic advanced oxidation method for treating organic pollutants. The study of electrode material modification using nano ceria has received widespread attention both domestically and internationally. Researchers mainly introduce nano cerium oxide and its composite materials through different chemical methods to modify different electrode materials, improve their electrochemical activity, and thereby increase electrocatalytic activity and final removal rate.
Microwave and ultrasound are often important auxiliary measures for the above catalytic models. Taking ultrasonic assistance as an example, using vibration sound waves with frequencies higher than 25kHz per second, millions of extremely small bubbles are generated in a solution formulated with a specially designed cleaning agent. These small bubbles, during rapid compression and expansion, constantly produce bubble implosion, allowing materials to quickly exchange and diffuse on the catalyst surface, often exponentially improving catalytic efficiency.
3 Conclusion
Nano ceria and its composite materials can effectively treat ions and organic pollutants in water, and have important application potential in future water treatment fields. However, most research is still in the laboratory stage, and in order to achieve rapid application in water treatment in the future, the following issues still need to be urgently addressed:
(1) The relatively high preparation cost of nano CeO2 based materials remains an important factor in the vast majority of their applications in water treatment, which are still in the laboratory research stage. Exploring low-cost, simple and effective preparation methods that can regulate the morphology and size of nano CeO2 based materials is still a focus of research.
(2) Due to the small particle size of nano CeO2 based materials, the recycling and regeneration issues after use are also important factors limiting their application. The composite of it with resin materials or magnetic materials will be a key research direction for its material preparation and recycling technology.
(3) The development of a joint process between nano CeO2 based material water treatment technology and traditional sewage treatment technology will greatly promote the application of nano CeO2 based material catalytic technology in the field of water treatment.
(4) There is still limited research on the toxicity of nano CeO2 based materials, and their environmental behavior and toxicity mechanism in water treatment systems have not been determined yet. The actual sewage treatment process often involves the coexistence of multiple pollutants, and the coexisting pollutants will interact with each other, thereby changing the surface characteristics and potential toxicity of nanomaterials. Therefore, there is an urgent need to carry out more research on related aspects.
Post time: May-22-2023